US FDA LAAF Recognition

EUROFINS India Makes History with FDA LAAF Recognition: A Game-Changer for Food Safety
In a groundbreaking achievement, EUROFINS Analytical Services India, based in Bengaluru, has become the first laboratory in Asia to earn recognition under the U.S. Food and Drug Administration’s (FDA) Laboratory Accreditation for Analyses of Foods (LAAF) program. This milestone not only highlights EUROFINS’ commitment to excellence but also reinforces its pivotal role in ensuring the safety and quality of food consumed in the United States. Let’s dive into what this means, why it matters, and how EUROFINS is setting a new standard for global food testing.

A Milestone for Food Safety: What is the LAAF Program?
The LAAF program, established under the Food Safety Modernization Act (FSMA), is the FDA’s gold standard for accrediting laboratories that test foods regulated by the agency. It is designed to ensure that food testing is conducted with precision, reliability, and scientific rigor to protect public health. By achieving LAAF recognition, EUROFINS India has proven its ability to meet stringent international standards, including ISO/IEC 17025:2017 and the FDA’s LAAF-specific requirements.
Why is this a big deal?
LAAF accreditation guarantees that testing results are accurate and trustworthy, helping prevent foodborne illnesses and ensuring that food products meet the highest safety and quality standards. For EUROFINS India, this recognition is a testament to its cutting-edge facilities, expert team, and unwavering dedication to excellence.
Eurofins India: The First LAAF-Recognized Laboratory in Asia
Eurofins India has achieved this recognition after rigorous assessments and audits, demonstrating full compliance with

- ISO/IEC 17025:2017 — International standard for laboratory quality and competence.
- AC89 (IAS Accreditation Criteria) — Specific criteria required by International Accreditation Service (IAS).
- FDA LAAF Final Rule — Defining technical and operational requirements under FSMA.
This recognition reinforces our global reputation for scientific excellence, quality assurance, and compliance leadership.
— Eurofins India Management
The FDA and FSMA: Guardians of Food Safety
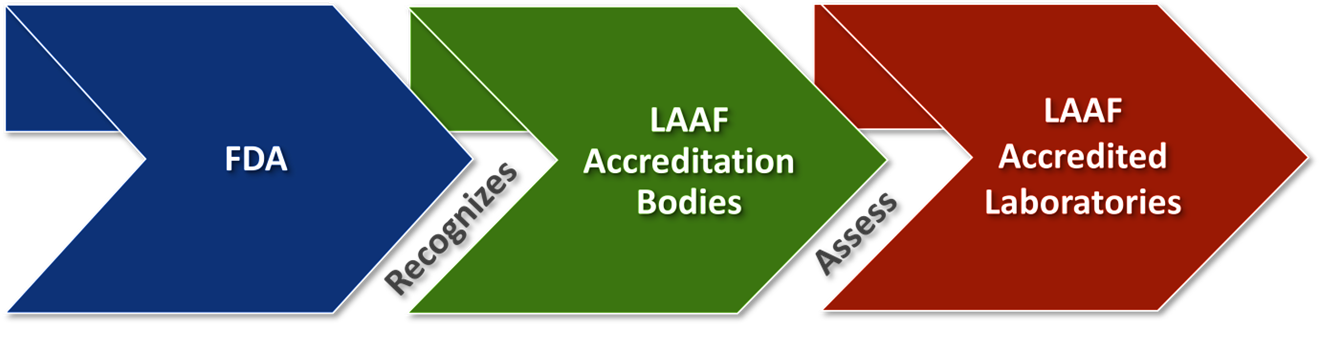
The U.S. Food and Drug Administration (FDA) is a global leader in protecting public health by regulating food, drugs, medical devices, and cosmetics. Its Food Safety Modernization Act (FSMA), enacted in 2011, revolutionized food safety by shifting the focus from reacting to foodborne outbreaks to preventing them. FSMA mandates science-based preventive controls, such as hazard analysis and risk-based measures, to identify and eliminate potential food safety risks.
The LAAF program is a cornerstone of FSMA, ensuring that laboratories testing FDA-regulated foods meet rigorous standards. By accrediting labs like EUROFINS India, the FDA strengthens its ability to safeguard the U.S. food supply.
How EUROFINS Earned LAAF Recognition
Achieving LAAF accreditation is no small feat. EUROFINS India underwent a rigorous process to prove its competence, including:
- Application to an FDA-Recognized Accreditation Body: EUROFINS applied to an accreditation body that evaluated its compliance with LAAF standards.
- Comprehensive Assessment: The accreditation body reviewed EUROFINS’ management systems, personnel qualifications, facilities, equipment, and quality assurance practices.
- Analytical Procedure Review: The lab’s testing methods and data handling practices were scrutinized to ensure accuracy and reliability.
- Ongoing Compliance: EUROFINS demonstrated adherence to international standards like ISO/IEC 17025 and participated in proficiency testing to maintain its accreditation.
This meticulous process ensures that EUROFINS delivers results that businesses and regulators can trust.
Global Standards with Local Execution
Being LAAF-accredited allows Eurofins India to offer globally recognized testing services for foods regulated by the FDA — including products intended for export to the U.S. This provides manufacturers with a significant edge in global trade.
Benefits for Manufacturers & Exporters
|
Advantage |
How It Helps Your Business |
|
FDA Recognition |
Trusted results for U.S. regulatory compliance and faster market access. |
|
Reduced Testing Delays |
No need for re-testing or foreign lab verifications. |
|
International Trade Acceptance |
Recognition under ILAC MRA ensures testing credibility worldwide. |
|
Competitive Business Advantage |
Preferred by buyers, importers, and regulators. |
|
Confidence in Results |
High accuracy, validated procedures, and regulatory traceability. |
Range of Testing Covered Under LAAF
The Laboratory Accreditation for Analyses of Foods (LAAF) program, established by the U.S. FDA under the Food Safety Modernization Act (FSMA), sets requirements for when food testing must be conducted by laboratories accredited to specific standards. The program aims to ensure the accuracy, reliability, and uniformity of food testing in certain regulatory and enforcement contexts.
Types of Testing Covered
The LAAF program covers a broad range of food testing methods, including:
- Chemical analyses (e.g., for pesticides, mycotoxins, food additives)
- Microbiological analyses (e.g., bacteria, yeast, mold)
- Radiological analyses
- Other biological and environmental tests (e.g., genetically modified organisms, water activity).
Why LAAF Accreditation Matters
The significance of Eurofins India’s LAAF recognition goes beyond regulatory compliance it demonstrates an unwavering commitment to food safety, innovation, and global collaboration.
Key Benefits of LAAF Accreditation
|
Benefit |
Impact |
|
Enhanced Public Health Protection |
Ensures only safe, compliant foods enter consumer markets. |
|
Credibility & Trust |
Builds confidence among clients, authorities, and international buyers. |
|
Continuous Improvement |
Drives innovation and improvement in laboratory procedures and systems. |
|
Global Recognition |
Meets standards required by many importing countries and global brands. |
|
Client Confidence |
Businesses choose accredited labs to validate their supply chain quality. |
Eurofins services
Accreditation: ISO/IEC 17025:2017
Effective Date: March 23, 2023
Accrediting Body: U.S. Food and Drug Administration (FDA)
Program: Laboratory Accreditation for Analyses of Foods (LAAF)
Eurofins Analytical Services India, based in Bengaluru, has been officially recognized under the FDA’s LAAF program. The laboratory is accredited to perform both chemical and biological analyses on a wide range of food and food-related products using validated international methods.
Field of Testing: Chemical
|
Material / Matrix |
Analyte(s) |
Method Reference |
|
Alimentary Pastes |
Light Filth |
AOAC 969.41 – Light Filth in Alimentary Pastes |
|
Spices & Condiments |
Heavy and Light Filth |
AOAC 975.48 – Heavy and Light Filth in Spices |
|
Whole Wheat Flour |
Light Filth |
AOAC 993.26 – Light Filth in Whole Wheat Flour |
|
Food & Food Products* |
Cadmium, Lead, Arsenic, Mercury |
AOAC 2015.01 – Heavy Metals in Food (ICP-MS Method) |
*Includes: Rice, Guar gum, Infant food, Nutritional supplements, Nutraceuticals, Oil seeds & nuts, Herbs, Condiments & spices, Processed & canned foods, Ready-to-eat foods, Honey, Shrimp, Prawn, Bakery & Confectionery
Field of Testing: Biological
|
Material / Matrix |
Microorganism(s) |
Method Reference |
|
Food & Food Products* |
Escherichia coli |
ISO 16649-2 – Enumeration of β-glucuronidase-positive E. coli (Colony Count, 44°C) |
|
Salmonella |
US FDA BAM Chapter 5 – Salmonella Detection |
|
|
Listeria monocytogenes |
ISO 11290-1 – Detection & Enumeration of Listeria monocytogenes |
Setting a New Standard for Indian Food Testing
 Eurofins India’s LAAF recognition is more than a certification — it is a strategic asset for food manufacturers, exporters, and importers who need precise, fast, and reliable testing backed by global regulatory approval.
Eurofins India’s LAAF recognition is more than a certification — it is a strategic asset for food manufacturers, exporters, and importers who need precise, fast, and reliable testing backed by global regulatory approval.
Whether you are targeting the U.S. market, seeking brand protection, or strengthening your food safety protocols, Eurofins is your trusted partner.
